News & Headlines
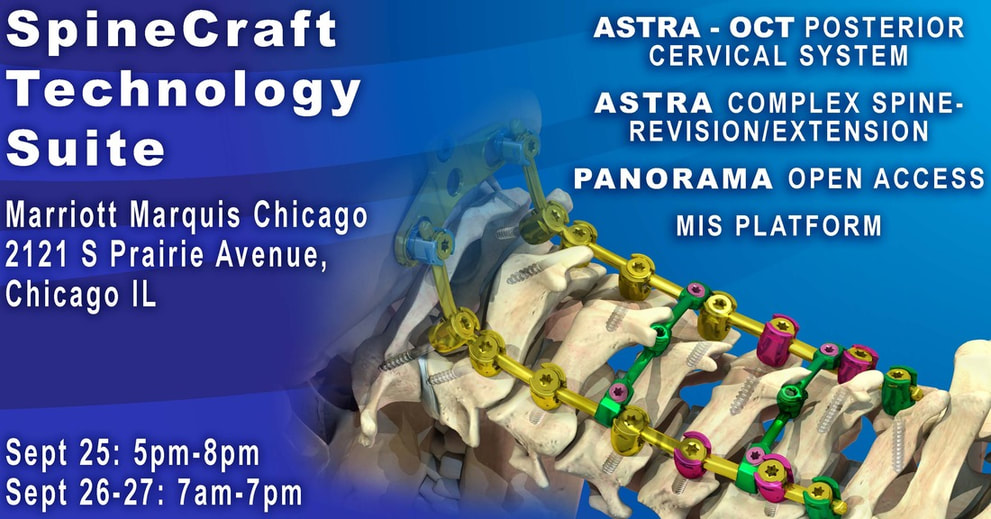
September 05, 2019 | Visit SpineCraft Technology Suite in Chicago, IL. September 25-27
Visit the SpineCraft Technology Suite at:
Mariott Marquis Chicago
2121 S Prairie Avenue,
Chicago IL
Sept 25: 5PM - 8PM
Sept 26-27: 7AM - 7PM
Featured will be the ASTRA GLOBAL SPINE SYSTEM including the introduction of the ASTRA-OCT Posterior Cervical System
ASTRA GLOBAL SPINE SYSTEM
Also featured will be the PANORAMA Open-Access MIS retractor
Stop Fighting the Tube
Contact SpineCraft for details
Visit the SpineCraft Technology Suite at:
Mariott Marquis Chicago
2121 S Prairie Avenue,
Chicago IL
Sept 25: 5PM - 8PM
Sept 26-27: 7AM - 7PM
Featured will be the ASTRA GLOBAL SPINE SYSTEM including the introduction of the ASTRA-OCT Posterior Cervical System
ASTRA GLOBAL SPINE SYSTEM
- Occiput to Pelvis
- Complex Spine & Deformity
- Comprehensive Implant Offering
- Revision & Extension Module
- Pelvic Fixation including S2AI offering
- POWER MODULE
- PSO instrumentation
- Surgical Planning and Mechanical Guidance
- MIS platform
Also featured will be the PANORAMA Open-Access MIS retractor
Stop Fighting the Tube
Contact SpineCraft for details
April 16, 2019 | SpineCraft Introduces ASTRA SPINE SYSTEM POWER Instrumentation for Deformity & Complex Spine Procedures
SpineCraft announces the introduction of the ASTRA SPINE SYSTEM- POWER INSTRUMENTATION Module. The ASTRA SPINE SYSTEM was introduced in early 2017 and includes a comprehensive implant and instrument offering for complex spine, deformity, degenerative and MIS Spine procedures.
The ASTRA POWER INSTRUMENTATION includes taps and drivers that are uniquely designed to improve surgical ergonomics, reduce operating fatigue and provide better precision when implanting spinal implants.
Mark Moran MD., Pediatric Orthopedic Surgery, Boca Raton, Florida
“When correcting spinal deformity in young patients, it is our goal to achieve the best possible correction while simultaneously reducing the overall time of the procedure. To accomplish this, we utilize a surgical technique that includes ASTRA power instrumentation that is designed to work within the tight confines of intraoperative imaging. We have found that this approach enhances implant placement accuracy while reducing operating time. We are very pleased with this technique and how our patients have fared”.
SpineCraft announces the introduction of the ASTRA SPINE SYSTEM- POWER INSTRUMENTATION Module. The ASTRA SPINE SYSTEM was introduced in early 2017 and includes a comprehensive implant and instrument offering for complex spine, deformity, degenerative and MIS Spine procedures.
The ASTRA POWER INSTRUMENTATION includes taps and drivers that are uniquely designed to improve surgical ergonomics, reduce operating fatigue and provide better precision when implanting spinal implants.
Mark Moran MD., Pediatric Orthopedic Surgery, Boca Raton, Florida
“When correcting spinal deformity in young patients, it is our goal to achieve the best possible correction while simultaneously reducing the overall time of the procedure. To accomplish this, we utilize a surgical technique that includes ASTRA power instrumentation that is designed to work within the tight confines of intraoperative imaging. We have found that this approach enhances implant placement accuracy while reducing operating time. We are very pleased with this technique and how our patients have fared”.
September 10, 2018 | SpineCraft Presents at SMISS Conference in Las Vegas
SpineCraft attended the 2018 SMISS conference in Las Vegas. At the conference, Adam Lewis MD and Steve Mather MD introduced the SpineCraft PANORAMA retractor and STAC MIS technique during the new technology forum. The presentation included a review of the two-year history of the STAC MIS technique and the surgical benefits offered by the SpineCraft PANORAMA retractor.
SpineCraft attended the 2018 SMISS conference in Las Vegas. At the conference, Adam Lewis MD and Steve Mather MD introduced the SpineCraft PANORAMA retractor and STAC MIS technique during the new technology forum. The presentation included a review of the two-year history of the STAC MIS technique and the surgical benefits offered by the SpineCraft PANORAMA retractor.
July 6, 2018 | SpineCraft Receives FDA 510(k) Clearance for the ASTRA-OCT System
SpineCraft is pleased to announce their ASTRA-OCT (occipito-cervico-thoracic) system has received 510(k) clearance. This comprehensive system offers numerous advantages, including extensive revision & extension capabilities and ability to accommodate multiple rod diameters & materials.
The ASTRA-OCT System is an important addition to SpineCraft’s robust Deformity and Complex Spine portfolio.
SpineCraft is pleased to announce their ASTRA-OCT (occipito-cervico-thoracic) system has received 510(k) clearance. This comprehensive system offers numerous advantages, including extensive revision & extension capabilities and ability to accommodate multiple rod diameters & materials.
The ASTRA-OCT System is an important addition to SpineCraft’s robust Deformity and Complex Spine portfolio.
June 6, 2018 | SpineCraft to Participate in Becker's 16th Annual Future of Spine + The Spine, Orthopedic and Pain Management-Driven ASC Conference
We hope you will visit the SpineCraft table at Becker's 16th Annual Future of Spine + The Spine, Orthopedic and Pain Management-Driven ASC Conference at the Swissotel in Chicago, Illinois, June 14-16. Stop by booth #20 to learn more about our products.
The sessions cover a range of high-level discussions on strategy mixed with on-the-ground observations from the spine, orthopedic and pain management fields focusing on the outpatient setting. The sessions include a range of differing perspectives and critical insight on healthcare, business, economic, clinical and political trends.
We hope you will visit the SpineCraft table at Becker's 16th Annual Future of Spine + The Spine, Orthopedic and Pain Management-Driven ASC Conference at the Swissotel in Chicago, Illinois, June 14-16. Stop by booth #20 to learn more about our products.
The sessions cover a range of high-level discussions on strategy mixed with on-the-ground observations from the spine, orthopedic and pain management fields focusing on the outpatient setting. The sessions include a range of differing perspectives and critical insight on healthcare, business, economic, clinical and political trends.
May 3, 2018 | SpineCraft to Participate in the 2018 Castellvi Spine Conference
We hope you will visit the SpineCraft table at Castellvi Spine in Key West, Florida, May 10th to the 12th. Stop by booth #19 to learn more about our products.
This CME activity is designed for surgeons and non-operative clinicians with specific interest in disorders of the spine. Distinguished faculty will present the latest advances in the field including complex reconstruction, artificial disc replacement, use of biologics, spine biomechanics, management of complications, treatment modalities for osteoporosis, and emerging technologies.
We hope you will visit the SpineCraft table at Castellvi Spine in Key West, Florida, May 10th to the 12th. Stop by booth #19 to learn more about our products.
This CME activity is designed for surgeons and non-operative clinicians with specific interest in disorders of the spine. Distinguished faculty will present the latest advances in the field including complex reconstruction, artificial disc replacement, use of biologics, spine biomechanics, management of complications, treatment modalities for osteoporosis, and emerging technologies.
April 6, 2018 | SpineCraft to Participate in the 18th Annual ISASS Conference
We hope you will visit the SpineCraft exhibit at ISASS in Toronto, Ontario, Canada, April 11th to the 13th. Stop by booth #404 to learn more about our products.
The Annual Conference provides state-of-the-art continuing medical education and the perfect venue to learn about the latest products and services in spinal surgery and care. The educational elements of the meeting program are targeted to all spine surgery medical professionals and other professionals involved in spine surgery.
Download as PDF
We hope you will visit the SpineCraft exhibit at ISASS in Toronto, Ontario, Canada, April 11th to the 13th. Stop by booth #404 to learn more about our products.
The Annual Conference provides state-of-the-art continuing medical education and the perfect venue to learn about the latest products and services in spinal surgery and care. The educational elements of the meeting program are targeted to all spine surgery medical professionals and other professionals involved in spine surgery.
Download as PDF
March 3, 2018 | SpineCraft to Participate in Spine Summit 2018
Visit the SpineCraft booth, #315, at the Spine Summit 2018, March 14-17 in Universal Orlando, Florida.
Featured Product:
The patented PANORAMA Retractor System for minimally invasive & microdiscectomy spine procedures.
The patented PANORAMA slotted retractor is the first tubular retractor that includes an axial opening for greater working freedom in the disc space. The slotted design allows increased instrument angulation and versatility and expands the surgeon’s ability to safely access the entire disc space, including difficult to reach contralateral locations.
Panorama opens the confines of a cylindrical retractor and improves the efficiency and effectiveness of intervertebral discectomy and endplate preparation for fusion.
Visit the SpineCraft booth, #315, at the Spine Summit 2018, March 14-17 in Universal Orlando, Florida.
Featured Product:
The patented PANORAMA Retractor System for minimally invasive & microdiscectomy spine procedures.
The patented PANORAMA slotted retractor is the first tubular retractor that includes an axial opening for greater working freedom in the disc space. The slotted design allows increased instrument angulation and versatility and expands the surgeon’s ability to safely access the entire disc space, including difficult to reach contralateral locations.
Panorama opens the confines of a cylindrical retractor and improves the efficiency and effectiveness of intervertebral discectomy and endplate preparation for fusion.
February 21, 2018 | SpineCraft to Participate in the 69th Southern Neurological Society Annual Meeting
Come visit SpineCraft, table #51, at the 69th Southern Neurological Society (SNS) Annual Meeting, February 28-March 3 in Marco Island, Florida.
Featured Products:
AVANT MIS Spine System. This comprehensive thoracolumbar percutaneous system includes a wide variety of implant options as well as a patent-pending RCDF sleeve technology that condenses 5 instruments into one universal device.
VELOX Cervical Plating System. The VELOX plate includes multiple implant options and offers a high degree of screw angulation. This increased angulation allows the surgeon to use shorter plates with maximum screw fixation.
ASTRA Spine System is designed for spinal deformity correction as well as tumor, trauma and degenerative procedures. The ASTRA Spine System also includes a revision & extension module developed for the expanding revision patient population. The revision & extension module includes a variety of proprietary rods and linkages specifically designed to allow the surgeon to attach to pre-existing implants. This ability to link to the existing implants can reduce surgery time and the overall cost associated with a hardware removal and replacement.
The ASTRA Spine System is designed for use with 6.0 mm and 5.5 mm titanium and cobalt chromium rods. All rods are interchangeable within the same implants and instrumentation providing the surgeon valuable intraoperative flexibility while reducing the hospital inventory requirements. In addition, the ASTRA Spine System includes several implant enhancements including a favored angle design incorporated in all thoracolumbar polyaxial screws.
Come visit SpineCraft, table #51, at the 69th Southern Neurological Society (SNS) Annual Meeting, February 28-March 3 in Marco Island, Florida.
Featured Products:
AVANT MIS Spine System. This comprehensive thoracolumbar percutaneous system includes a wide variety of implant options as well as a patent-pending RCDF sleeve technology that condenses 5 instruments into one universal device.
VELOX Cervical Plating System. The VELOX plate includes multiple implant options and offers a high degree of screw angulation. This increased angulation allows the surgeon to use shorter plates with maximum screw fixation.
ASTRA Spine System is designed for spinal deformity correction as well as tumor, trauma and degenerative procedures. The ASTRA Spine System also includes a revision & extension module developed for the expanding revision patient population. The revision & extension module includes a variety of proprietary rods and linkages specifically designed to allow the surgeon to attach to pre-existing implants. This ability to link to the existing implants can reduce surgery time and the overall cost associated with a hardware removal and replacement.
The ASTRA Spine System is designed for use with 6.0 mm and 5.5 mm titanium and cobalt chromium rods. All rods are interchangeable within the same implants and instrumentation providing the surgeon valuable intraoperative flexibility while reducing the hospital inventory requirements. In addition, the ASTRA Spine System includes several implant enhancements including a favored angle design incorporated in all thoracolumbar polyaxial screws.
January 10, 2018 | SpineCraft Receives U.S. Patent for Spinal Derotation System & Methods
SpineCraft is pleased to announce their portfolio of issued patents has expanded. We have received a U.S. patent for the systems, assemblies and methods for spinal derotation.
SpineCraft offers comprehensive solutions for deformity surgeries requiring derotation. Options are available in both the ASTRA Deformity and APEX VCD spine systems.
SpineCraft is pleased to announce their portfolio of issued patents has expanded. We have received a U.S. patent for the systems, assemblies and methods for spinal derotation.
SpineCraft offers comprehensive solutions for deformity surgeries requiring derotation. Options are available in both the ASTRA Deformity and APEX VCD spine systems.
October 27, 2017 | SpineCraft to Participate in the 3rd Annual International Spinal Deformity Symposium
Visit the SpineCraft table at the 3rd Annual International Spinal Deformity Symposium (ISDS), at the Swissotel in Chicago, Illinois, November 3-4.
Featured Product:
The ASTRA Spine System is designed for spinal deformity correction as well as tumor, trauma and degenerative procedures. The ASTRA Spine System also includes revision & extension module developed for the expanding revision patient population. The revision & extension module includes a variety of proprietary rods and linkages specifically designed to allow the surgeon to attach to pre-existing implants. This ability to link to the existing implants can reduce surgery time and the overall cost associated with a hardware removal and replacement.
The ASTRA Spine System is designed for use with 6.0 mm and 5.5 mm titanium and cobalt chromium rods. All rods are interchangeable within the same implants and instrumentation providing the surgeon valuable intraoperative flexibility while reducing the hospital inventory requirements. In addition, the ASTRA Spine System includes several implant enhancements including a favored angle design incorporated in all thoracolumbar polyaxial screws.
Visit the SpineCraft table at the 3rd Annual International Spinal Deformity Symposium (ISDS), at the Swissotel in Chicago, Illinois, November 3-4.
Featured Product:
The ASTRA Spine System is designed for spinal deformity correction as well as tumor, trauma and degenerative procedures. The ASTRA Spine System also includes revision & extension module developed for the expanding revision patient population. The revision & extension module includes a variety of proprietary rods and linkages specifically designed to allow the surgeon to attach to pre-existing implants. This ability to link to the existing implants can reduce surgery time and the overall cost associated with a hardware removal and replacement.
The ASTRA Spine System is designed for use with 6.0 mm and 5.5 mm titanium and cobalt chromium rods. All rods are interchangeable within the same implants and instrumentation providing the surgeon valuable intraoperative flexibility while reducing the hospital inventory requirements. In addition, the ASTRA Spine System includes several implant enhancements including a favored angle design incorporated in all thoracolumbar polyaxial screws.
September 19, 2017 | SpineCraft Receives U.S. Patent for ALLTUM Surgical Plate System & Methods
SpineCraft is pleased to announce their portfolio of issued patents has expanded. We have received a U.S. patent for the surgical plate system and methods for ALLTUM Anterior Cervical Plate System.
SpineCraft offers uniquely designed anterior cervical plate systems which reduce “fiddle-factor” and increase efficiency. Options are available in both the ALLTUM and VELOX Cervical Plate Systems.
SpineCraft is pleased to announce their portfolio of issued patents has expanded. We have received a U.S. patent for the surgical plate system and methods for ALLTUM Anterior Cervical Plate System.
SpineCraft offers uniquely designed anterior cervical plate systems which reduce “fiddle-factor” and increase efficiency. Options are available in both the ALLTUM and VELOX Cervical Plate Systems.
August 1, 2017 | SpineCraft to Participate in the Tennessee Neurological Society Annual Meeting
We hope you will Visit the SpineCraft table at the Tennessee Neurological Society (TNS) Annual Meeting in Nashville, August 11-12.
The TNS is dedicated to serving and uniting Tennessee neurosurgeons. By standing together, they hope to improve neurosurgical care for our patients in our state and beyond – leading by example throughout the state, the nation, and the world.
We hope you will Visit the SpineCraft table at the Tennessee Neurological Society (TNS) Annual Meeting in Nashville, August 11-12.
The TNS is dedicated to serving and uniting Tennessee neurosurgeons. By standing together, they hope to improve neurosurgical care for our patients in our state and beyond – leading by example throughout the state, the nation, and the world.
June 8, 2017 | SpineCraft Receives U.S. Patent for Spinal Derotation System & Methods
SpineCraft is pleased to announce their portfolio of issued patents has expanded. We have received a U.S. patent for the systems, assemblies and methods for spinal derotation.
SpineCraft offers comprehensive solutions for deformity surgeries requiring derotation. Options are available in both the ASTRA Deformity and APEX VCD Spine Systems.
SpineCraft is pleased to announce their portfolio of issued patents has expanded. We have received a U.S. patent for the systems, assemblies and methods for spinal derotation.
SpineCraft offers comprehensive solutions for deformity surgeries requiring derotation. Options are available in both the ASTRA Deformity and APEX VCD Spine Systems.
May 23, 2017 | SpineCraft to Participate in the 12th Annual UCSF Spine Symposium
We hope you will visit the SpineCraft table at the 12th Annual UCSF Spine Symposium in San Francisco, California on June 2nd and June 3rd.
The UCSF Spine Symposium is an annual two-day event emphasizing pioneering trends in diagnostic and therapeutic strategies for patients suffering from spinal disorders.
This course is designed to be interactive with talks given by leaders in the spine community. All lectures are followed by case discussions aimed at highlighting key issues in breakthrough treatments.
The course is designed for neurosurgeons, orthopedists, nurses, physical therapists, physiatrists, anesthesiologists, pain specialists as well as primary care providers.
Download as PDF
We hope you will visit the SpineCraft table at the 12th Annual UCSF Spine Symposium in San Francisco, California on June 2nd and June 3rd.
The UCSF Spine Symposium is an annual two-day event emphasizing pioneering trends in diagnostic and therapeutic strategies for patients suffering from spinal disorders.
This course is designed to be interactive with talks given by leaders in the spine community. All lectures are followed by case discussions aimed at highlighting key issues in breakthrough treatments.
The course is designed for neurosurgeons, orthopedists, nurses, physical therapists, physiatrists, anesthesiologists, pain specialists as well as primary care providers.
Download as PDF
April 3, 2017 | Thank you for visiting SpineCraft at the 2017 AANS/CNS exhibit
We hope you visited the SpineCraft booth at the 2017 AANS/CNS Spine Summit in Las Vegas, Nevada on March 8th through March 11th.
We enjoyed introducing you to the AVANT MIS Spine System, along with our other products.
Download as PDF
We hope you visited the SpineCraft booth at the 2017 AANS/CNS Spine Summit in Las Vegas, Nevada on March 8th through March 11th.
We enjoyed introducing you to the AVANT MIS Spine System, along with our other products.
Download as PDF
February 23, 2017 | SpineCraft Receives U.S. Patent for Uniplanar Surgical Screw Assembly
SpineCraft is pleased to announce their portfolio of issued patents has expanded. We have received a U.S. patent for the Uniplanar Surgical Screw Assembly.
The Uniplanar Surgical Screw Assembly offers more flexibility for rod placement and is available in both the ASTRA and APEX Spine Systems.
SpineCraft is pleased to announce their portfolio of issued patents has expanded. We have received a U.S. patent for the Uniplanar Surgical Screw Assembly.
The Uniplanar Surgical Screw Assembly offers more flexibility for rod placement and is available in both the ASTRA and APEX Spine Systems.
February 2, 2017 | FireFly Mechanical Surgical Guidance System
SpineCraft LLC is pleased to announce that it has entered into an agreement with Mighty Oak Medical to use the FireFly Mechanical Surgical Guidance System with SpineCraft ASTRA & APEX Spine Systems for deformity correction and complex spine surgeries.
The Firefly system includes a CT-based surgical planning guide that enables the surgeon to pre-select pedicle screw trajectories along with implant sizes. Based on the surgical plan, the FireFly System 3D prints bilateral segmental drill guides that affix to the patient’s bone and mirror the surgical plan trajectory. This combination of pre-surgical planning and mechanical guidance allow for extremely accurate screw placement which is especially valuable on challenging complex spine procedures.
The FireFly-ASTRA & FireFly-APEX Systems can also greatly improve operating theater efficiency though the delivery of a patient specific implant tray configuration. By way of the planning software SpineCraft can deliver the preselected implants arranged in sequential order in a patient specific implant tray. The patient specific implant tray can improve surgical flow and significantly reduce the amount of implant inventory historically required for large spine surgeries.
Download as PDF
SpineCraft LLC is pleased to announce that it has entered into an agreement with Mighty Oak Medical to use the FireFly Mechanical Surgical Guidance System with SpineCraft ASTRA & APEX Spine Systems for deformity correction and complex spine surgeries.
The Firefly system includes a CT-based surgical planning guide that enables the surgeon to pre-select pedicle screw trajectories along with implant sizes. Based on the surgical plan, the FireFly System 3D prints bilateral segmental drill guides that affix to the patient’s bone and mirror the surgical plan trajectory. This combination of pre-surgical planning and mechanical guidance allow for extremely accurate screw placement which is especially valuable on challenging complex spine procedures.
The FireFly-ASTRA & FireFly-APEX Systems can also greatly improve operating theater efficiency though the delivery of a patient specific implant tray configuration. By way of the planning software SpineCraft can deliver the preselected implants arranged in sequential order in a patient specific implant tray. The patient specific implant tray can improve surgical flow and significantly reduce the amount of implant inventory historically required for large spine surgeries.
Download as PDF
January 16, 2017 | SpineCraft EU Approvals for ASTRA Spine System and VELOX ACP
SpineCraft has received recently the European CE Mark approvals for its ASTRA Spine System and VELOX Anterior Cervical Plate System. Both systems are already 510(K) cleared by the U.S. Food and Drug Administration (FDA).
Download as PDF
SpineCraft has received recently the European CE Mark approvals for its ASTRA Spine System and VELOX Anterior Cervical Plate System. Both systems are already 510(K) cleared by the U.S. Food and Drug Administration (FDA).
Download as PDF
September 26, 2016 | Visit SpineCraft at the 2016 SMISS Annual Forum at Booth #401
SpineCraft is honored to be a participating exhibitor at the 2016 SMISS Annual Forum on October 13-15th.
Come by the SpineCraft Booth #401 to see the new AVANT MIS Tab System with RCDF sleeve along with the many other SpineCraft products, including our AIM MIS system and our VELOX and ALTUM cervical plates.
We look forward to seeing you in Las Vegas!
Download as PDF
SpineCraft is honored to be a participating exhibitor at the 2016 SMISS Annual Forum on October 13-15th.
Come by the SpineCraft Booth #401 to see the new AVANT MIS Tab System with RCDF sleeve along with the many other SpineCraft products, including our AIM MIS system and our VELOX and ALTUM cervical plates.
We look forward to seeing you in Las Vegas!
Download as PDF
July 26, 2016 | SpineCraft Announces the Successful US Release of the ASTRA SPINE SYSTEM
SpineCraft would like to announce the successful US release of the ASTRA SPINE SYSTEM. The ASTRA SPINE SYSTEM is designed for spinal deformity correction as well as tumor, trauma and degenerative procedures. It also includes a new revision & extension module developed for the expanding revision patient population. The revision & extension module includes a variety of proprietary rods and linkages specifically designed to allow the surgeon to attach to pre-existing implants. This ability to link to the existing implants can reduce surgery time and the overall cost associated with a hardware removal and replacement.
The ASTRA SPINE SYSTEM is designed for use with 6.0 mm and 5.5 mm titanium and cobalt chromium rods. All rods are interchangeable within the same implants and instrumentation providing the surgeon valuable intraoperative flexibility while reducing the hospital inventory requirements. In addition, the ASTRA SPINE SYSTEM includes several implant enhancements including a favored angle design incorporated in all thoracolumbar polyaxial screws.
John K. Starr, MD of Washington Orthopedics & Sports Medicine and Clinical Professor of Orthopedic Surgery at George Washington University: “I began using the ASTRA SPINE SYSTEM in early 2016 and have found the system to be comprehensive and an excellent choice for my complex spine procedures. Initially I did not anticipate the benefit of the ASTRA favored-angle polyaxial screw. With multiple cases performed, I now recognize that the extreme favored angle helps facilitate rod placement and proper rod positioning. This advantage is particularly valuable with severe spinal rotation or scoliotic curves. The beauty of the ASTRA favored angle option is that you don’t have to ask for it; it’s built into every thoracolumbar screw and is there when you need it most.”
Download as PDF
SpineCraft would like to announce the successful US release of the ASTRA SPINE SYSTEM. The ASTRA SPINE SYSTEM is designed for spinal deformity correction as well as tumor, trauma and degenerative procedures. It also includes a new revision & extension module developed for the expanding revision patient population. The revision & extension module includes a variety of proprietary rods and linkages specifically designed to allow the surgeon to attach to pre-existing implants. This ability to link to the existing implants can reduce surgery time and the overall cost associated with a hardware removal and replacement.
The ASTRA SPINE SYSTEM is designed for use with 6.0 mm and 5.5 mm titanium and cobalt chromium rods. All rods are interchangeable within the same implants and instrumentation providing the surgeon valuable intraoperative flexibility while reducing the hospital inventory requirements. In addition, the ASTRA SPINE SYSTEM includes several implant enhancements including a favored angle design incorporated in all thoracolumbar polyaxial screws.
John K. Starr, MD of Washington Orthopedics & Sports Medicine and Clinical Professor of Orthopedic Surgery at George Washington University: “I began using the ASTRA SPINE SYSTEM in early 2016 and have found the system to be comprehensive and an excellent choice for my complex spine procedures. Initially I did not anticipate the benefit of the ASTRA favored-angle polyaxial screw. With multiple cases performed, I now recognize that the extreme favored angle helps facilitate rod placement and proper rod positioning. This advantage is particularly valuable with severe spinal rotation or scoliotic curves. The beauty of the ASTRA favored angle option is that you don’t have to ask for it; it’s built into every thoracolumbar screw and is there when you need it most.”
Download as PDF
July 5, 2016 | SpineCraft Obtains Regulatory Clearance to market APEX Spine System® in China
SpineCraft announced today that it has received clearance from the China Food and Drug Administration (CFDA) to market its APEX Spine System® in China. The APEX Spine System is designed for complex deformity correction as well as tumor, trauma and degenerative procedures and has proven clinical track record with over 15,000 surgeries performed since its launch in the US and internationally.
SpineCraft also received clearance from the CFDA to market its AIM MIS System®, a minimally invasive posterior fixation solution addressing disorders in the thoracic and lumbar spine via a minimally invasive surgical procedure. The AIM MIS System has been in use in other markets since 2012.
“This CFDA clearance is a significant milestone achievement for SpineCraft is an important step in expanding the global distribution of our spine systems and further advances our strategy to establish SpineCraft as a true global spine company. The Chinese market in particular represents an important growing opportunity for SpineCraft products. The approval of APEX and AIM systems opens the door to participate in public tenders and expand our product offer proposition to include governmental and military hospitals" said SpineCraft CEO Wagdy Asaad, MD.
The China spine market has become the second most important after the USA and is projected to be worth over $1 billion by 2019. This is driven by China’s large population, rising standard of living, improving treatment options and growing ageing population; all signal significant room for growth.
Download as PDF
SpineCraft announced today that it has received clearance from the China Food and Drug Administration (CFDA) to market its APEX Spine System® in China. The APEX Spine System is designed for complex deformity correction as well as tumor, trauma and degenerative procedures and has proven clinical track record with over 15,000 surgeries performed since its launch in the US and internationally.
SpineCraft also received clearance from the CFDA to market its AIM MIS System®, a minimally invasive posterior fixation solution addressing disorders in the thoracic and lumbar spine via a minimally invasive surgical procedure. The AIM MIS System has been in use in other markets since 2012.
“This CFDA clearance is a significant milestone achievement for SpineCraft is an important step in expanding the global distribution of our spine systems and further advances our strategy to establish SpineCraft as a true global spine company. The Chinese market in particular represents an important growing opportunity for SpineCraft products. The approval of APEX and AIM systems opens the door to participate in public tenders and expand our product offer proposition to include governmental and military hospitals" said SpineCraft CEO Wagdy Asaad, MD.
The China spine market has become the second most important after the USA and is projected to be worth over $1 billion by 2019. This is driven by China’s large population, rising standard of living, improving treatment options and growing ageing population; all signal significant room for growth.
Download as PDF
June 21, 2016 | SpineCraft's global completion of 15,000 APEX Spine System Surgeries
The APEX Spine System is a comprehensive and versatile posterior instrumentation system designed for complex deformity correction as well as tumor, trauma and degenerative procedures. APEX is available in both the US and international marketplace and includes a wide range of implants and instrumentation that has made APEX a proven and reliable system for surgeons throughout the world.
APEX Spine System is designed for use with 6.0 mm and 5.5 mm titanium and cobalt chromium rods. All rods are interchangeable within the same implants and instrumentation providing the surgeon valuable intraoperative flexibility while reducing the hospital inventory requirements. In addition, APEX includes a wide-range of screw, hook and connector options.
Anis Mekhail MD-University of Illinois Hospital & Parkview Orthopaedic Group. “I have used APEX for several years and it has become my workhorse system for both complex spine as well as degenerative procedures. The broad range of implants and quality instrumentation allows me the surgical flexibility I require to address the most challenging procedures.”
Download as PDF
The APEX Spine System is a comprehensive and versatile posterior instrumentation system designed for complex deformity correction as well as tumor, trauma and degenerative procedures. APEX is available in both the US and international marketplace and includes a wide range of implants and instrumentation that has made APEX a proven and reliable system for surgeons throughout the world.
APEX Spine System is designed for use with 6.0 mm and 5.5 mm titanium and cobalt chromium rods. All rods are interchangeable within the same implants and instrumentation providing the surgeon valuable intraoperative flexibility while reducing the hospital inventory requirements. In addition, APEX includes a wide-range of screw, hook and connector options.
Anis Mekhail MD-University of Illinois Hospital & Parkview Orthopaedic Group. “I have used APEX for several years and it has become my workhorse system for both complex spine as well as degenerative procedures. The broad range of implants and quality instrumentation allows me the surgical flexibility I require to address the most challenging procedures.”
Download as PDF
March 11, 2016 | SpineCraft will attend the 2016 Spine Summit Conference
SpineCraft is honored to be a participating exhibitor at the 2016 AANS/CNS Spine Summit on March 16-19th. Visit us at Booth #415.
Please drop by our Booth #415 to see SpineCraft's products including the ASTRA Spine System for Complex Deformity and Revision Surgery as well as the AIM MIS Spine System and VELOX and ALTUM cervical plate.
Download as PDF
SpineCraft is honored to be a participating exhibitor at the 2016 AANS/CNS Spine Summit on March 16-19th. Visit us at Booth #415.
Please drop by our Booth #415 to see SpineCraft's products including the ASTRA Spine System for Complex Deformity and Revision Surgery as well as the AIM MIS Spine System and VELOX and ALTUM cervical plate.
Download as PDF
October 21, 2015 | Thank you for visiting SpineCraft at the NASS 2015
We enjoyed introducing you to the ASTRA Spine System for Deformity Correction and Complex Spine Procedures, along with our other products.
Download as PDF
We enjoyed introducing you to the ASTRA Spine System for Deformity Correction and Complex Spine Procedures, along with our other products.
Download as PDF
October 7, 2015 | SpineCraft Exhibiting at the NASS 2015
SpineCraft will be exhibiting at the NASS Annual Meeting in our hometown Chicago, IL on October 14-17, 2015.
We invite you to stop by Booth #1255 to meet members of our team and hear about our latest products and new system releases.
We look forward to seeing you in Chicago!
Download as PDF
SpineCraft will be exhibiting at the NASS Annual Meeting in our hometown Chicago, IL on October 14-17, 2015.
We invite you to stop by Booth #1255 to meet members of our team and hear about our latest products and new system releases.
We look forward to seeing you in Chicago!
Download as PDF
September 23, 2015 | SpineCraft at the 2015 SRS Annual Meeting
SpineCraft is proud to be a Diamond Level Supporter of the Scoliosis Research Society’s 50th Annual Meeting & Course in Minneapolis, September 30 - October 3, 2015.
We would like to extend an invitation to visit our private Diamond level supporter room (Director’s Row 1) on level 3 of the Hilton Minneapolis, near registration. Members of our product development and deformity product management team will be available to discuss the latest deformity correction and complex spine surgery solutions from SpineCraft and address any questions you may have.
Download as PDF
SpineCraft is proud to be a Diamond Level Supporter of the Scoliosis Research Society’s 50th Annual Meeting & Course in Minneapolis, September 30 - October 3, 2015.
We would like to extend an invitation to visit our private Diamond level supporter room (Director’s Row 1) on level 3 of the Hilton Minneapolis, near registration. Members of our product development and deformity product management team will be available to discuss the latest deformity correction and complex spine surgery solutions from SpineCraft and address any questions you may have.
Download as PDF
July 20, 2015 | Thank you for visiting SpineCraft at IMAST 2015
We hope you visited the SpineCraft booth at the 2015 IMAST 22nd International Meeting on Advanced Spine Techniques (IMAST), on July 8th - 11th in Kuala Lumpur, Malaysia.
Our Hands-on workshops included:
Download as PDF
We hope you visited the SpineCraft booth at the 2015 IMAST 22nd International Meeting on Advanced Spine Techniques (IMAST), on July 8th - 11th in Kuala Lumpur, Malaysia.
Our Hands-on workshops included:
- “Execution of Scoliosis Surgery: Implant Options, Rod Choices, Differential Rod Bending Techniques, and Direct Vertebral Rotation” presented by Dr. Kamal Ibrahim MD, FRCS(c), MA
- “Etiology and Surgical Management of Adjacent Segment Failure” presented by Dr. Hani Mhaidli MD, PhD
- “Techniques of Lumbopelvic and Sacral Fixation” presented by Dr. Hani Mhaidli MD, PhD
Download as PDF
May 19, 2015 | ASTRA Spine System FDA Clearance
SpineCraft, LLC, a privately-held US medical device company, recently announced that it has received regulatory clearance from the U.S. Food and Drug Administration (FDA) to market its ASTRA Spine System - a comprehensive posterior spinal fixation system.
The ASTRA Spine System is SpineCraft’s next generation deformity correction technology, designed by industry leading spine professionals to treat a range of pathologies efficiently and effectively. The system is optimized for use with advanced instrumentation developed specifically for complex spine procedures. The ASTRA Spine System implants and instruments are functional and precise, providing surgeons with exemplary reliability. The implants are designed with proven technology that allows intraoperative flexibility to choose rod diameter and material types while maintaining a low profile and providing exceptional strength.
The ASTRA Spine System is intended to provide immobilization and stabilization of spinal segments in skeletally mature patients as an adjunct to fusion in the treatment of the following acute and chronic instabilities or deformities of the thoracic, lumbar, and sacral spine; severe spondylolisthesis (grades 3 and 4) of the L5-S1 vertebra; degenerative spondylolisthesis with objective evidence of neurologic impairment; fracture; dislocation; scoliosis; kyphosis; spinal tumor; and failed previous fusion (pseudo-arthrosis).
Download as PDF
SpineCraft, LLC, a privately-held US medical device company, recently announced that it has received regulatory clearance from the U.S. Food and Drug Administration (FDA) to market its ASTRA Spine System - a comprehensive posterior spinal fixation system.
The ASTRA Spine System is SpineCraft’s next generation deformity correction technology, designed by industry leading spine professionals to treat a range of pathologies efficiently and effectively. The system is optimized for use with advanced instrumentation developed specifically for complex spine procedures. The ASTRA Spine System implants and instruments are functional and precise, providing surgeons with exemplary reliability. The implants are designed with proven technology that allows intraoperative flexibility to choose rod diameter and material types while maintaining a low profile and providing exceptional strength.
The ASTRA Spine System is intended to provide immobilization and stabilization of spinal segments in skeletally mature patients as an adjunct to fusion in the treatment of the following acute and chronic instabilities or deformities of the thoracic, lumbar, and sacral spine; severe spondylolisthesis (grades 3 and 4) of the L5-S1 vertebra; degenerative spondylolisthesis with objective evidence of neurologic impairment; fracture; dislocation; scoliosis; kyphosis; spinal tumor; and failed previous fusion (pseudo-arthrosis).
Download as PDF
February 27, 2015 | SpineCraft Exhibiting at AANS/CNS 2015
SpineCraft will be exhibiting at the AANS/CNS Spine Summit 2015 in Phoenix, AZ March 4th-7th. We invite you to stop by Booth #407 to meet members of our team and hear about our latest products and new system releases. SpineCraft will also be sponsoring a What’s New Session entitled:
“Tips & Techniques for MIS Screw Placement and Rod Insertion” presented by Dr. Anis Mekhail
We look forward to seeing you in Phoenix!
Download as PDF
SpineCraft will be exhibiting at the AANS/CNS Spine Summit 2015 in Phoenix, AZ March 4th-7th. We invite you to stop by Booth #407 to meet members of our team and hear about our latest products and new system releases. SpineCraft will also be sponsoring a What’s New Session entitled:
“Tips & Techniques for MIS Screw Placement and Rod Insertion” presented by Dr. Anis Mekhail
- Friday, March 6th @1:20pm
- Demonstration Theater
We look forward to seeing you in Phoenix!
Download as PDF
October 28, 2014 | October 2014 Newsletter
New: APEX Spine System Double Lead Thread Screws
The new APEX Spine System Double Lead Thread Screws are scheduled for release in select international markets in January 2015. Release in the US market is planned shortly after.
AVANT MIS System
The AVANT MIS System combines the advantages of a minimized surgical approach with the added benefit of simplified instrumentation and exceptional ergonomics. This system will feature a pre-attached Extended Screw Tulip, which guides rod and set screw insertion, eliminating the need for MIS tube assembly. With the ongoing hard work and dedication of our team, we are getting closer to the AVANT MIS System release date.
Hands-On Workshop
SpineCraft participated in the 3rd Spine Deformity Solutions Workshop: A Hands-On Course Presented by SRS in Burr Ridge, IL October 9-11. We presented our MIS lumbar TLIF and Percutaneous Pedicle Screws T12-S1 as well as our Open Lumbo-Sacral Techniques, Including: PSO, Sacro-Iliac Fixation.
Download as PDF
New: APEX Spine System Double Lead Thread Screws
The new APEX Spine System Double Lead Thread Screws are scheduled for release in select international markets in January 2015. Release in the US market is planned shortly after.
AVANT MIS System
The AVANT MIS System combines the advantages of a minimized surgical approach with the added benefit of simplified instrumentation and exceptional ergonomics. This system will feature a pre-attached Extended Screw Tulip, which guides rod and set screw insertion, eliminating the need for MIS tube assembly. With the ongoing hard work and dedication of our team, we are getting closer to the AVANT MIS System release date.
Hands-On Workshop
SpineCraft participated in the 3rd Spine Deformity Solutions Workshop: A Hands-On Course Presented by SRS in Burr Ridge, IL October 9-11. We presented our MIS lumbar TLIF and Percutaneous Pedicle Screws T12-S1 as well as our Open Lumbo-Sacral Techniques, Including: PSO, Sacro-Iliac Fixation.
Download as PDF
September 29, 2014 | September 2014 Newsletter
APEX Spine System’s New Double Lead Thread Screw Technology
We are preparing to release the new APEX Spine System screws with Dual Core and Double Lead Thread that provide distinctly optimized purchase for the proximal cortical bone of the pedicle as well as for the distal cancellous bone area. This design also allows fast and controlled screw insertion with outstanding pullout resistance.
SpineCraft at SRS - Anchorage, AK
SpineCraft participated at the SRS 49th Annual Meeting & Course in Anchorage this month with Diamond Level Support. We had a great time meeting many of you in our Hospitality Room and learning about the latest in spine technology. Our next SRS involvement will be at the upcoming Hands-On Workshop in Burr Ridge in October.
Hands-On Workshop
SpineCraft is preparing for participation in the 3rd Spine Deformity Solutions Workshop: A Hands-On Course Presented by SRS in Burr Ridge, IL October 9-11. We will have two lab sessions featuring: 1) MIS lumbar TLIF and Percutaneous Pedicle Screws T12-S1 and 2) Open Lumbo-Sacral Techniques, Including: PSO, Sacro-Iliac Fixation.
Download as PDF
APEX Spine System’s New Double Lead Thread Screw Technology
We are preparing to release the new APEX Spine System screws with Dual Core and Double Lead Thread that provide distinctly optimized purchase for the proximal cortical bone of the pedicle as well as for the distal cancellous bone area. This design also allows fast and controlled screw insertion with outstanding pullout resistance.
SpineCraft at SRS - Anchorage, AK
SpineCraft participated at the SRS 49th Annual Meeting & Course in Anchorage this month with Diamond Level Support. We had a great time meeting many of you in our Hospitality Room and learning about the latest in spine technology. Our next SRS involvement will be at the upcoming Hands-On Workshop in Burr Ridge in October.
Hands-On Workshop
SpineCraft is preparing for participation in the 3rd Spine Deformity Solutions Workshop: A Hands-On Course Presented by SRS in Burr Ridge, IL October 9-11. We will have two lab sessions featuring: 1) MIS lumbar TLIF and Percutaneous Pedicle Screws T12-S1 and 2) Open Lumbo-Sacral Techniques, Including: PSO, Sacro-Iliac Fixation.
Download as PDF
August 28, 2014 | August 2014 Newsletter
AVANT MIS System
The AVANT MIS System combines the advantages of a minimized surgical approach with the added benefit of simplified instrumentation and exceptional ergonomics. This system will feature a pre-attached Extended Screw Tulip, which guides rod and set screw insertion, eliminating the need for MIS tube assembly. With the ongoing hard work and dedication of our team, we are getting closer to the AVANT MIS System release date next quarter.
New APEX Spine System Cases
The new APEX Spine System implant & instrument case configurations that were released last July are now available worldwide! The new configuration includes several new instruments & implants and new tray/case layouts for OR convenience and streamlined processing.
Contact us for more information or to see our new brochure!
Hands-On Workshop
SpineCraft is preparing for participation in the 3rd Spine Deformity Solutions Workshop: A Hands-On Course Presented by SRS in Burr Ridge, IL October 9-11. We will have two lab sessions featuring: 1) MIS lumbar TLIF and Percutaneous Pedicle Screws T12-S1 and 2) Open Lumbo-Sacral Techniques, Including: PSO, Sacro-Iliac Fixation.
Download as PDF
AVANT MIS System
The AVANT MIS System combines the advantages of a minimized surgical approach with the added benefit of simplified instrumentation and exceptional ergonomics. This system will feature a pre-attached Extended Screw Tulip, which guides rod and set screw insertion, eliminating the need for MIS tube assembly. With the ongoing hard work and dedication of our team, we are getting closer to the AVANT MIS System release date next quarter.
New APEX Spine System Cases
The new APEX Spine System implant & instrument case configurations that were released last July are now available worldwide! The new configuration includes several new instruments & implants and new tray/case layouts for OR convenience and streamlined processing.
Contact us for more information or to see our new brochure!
Hands-On Workshop
SpineCraft is preparing for participation in the 3rd Spine Deformity Solutions Workshop: A Hands-On Course Presented by SRS in Burr Ridge, IL October 9-11. We will have two lab sessions featuring: 1) MIS lumbar TLIF and Percutaneous Pedicle Screws T12-S1 and 2) Open Lumbo-Sacral Techniques, Including: PSO, Sacro-Iliac Fixation.
Download as PDF
June 2, 2014 | SpineCraft Exhibiting at IMAST 2014
SpineCraft is a sponsor of the SRS with Diamond Level Support and will be exhibiting at the 2014 IMAST International Meeting.
We invite you to visit the SpineCraft booth at the 2014 IMAST 21st International Meeting on Advanced Spine Techniques, July 16th - 19th in Valencia, Spain, where we will have our latest spine solutions, a Hands-on Workshop, and expert staff on hand to answer your questions.
The Hands-on Workshop, entitled:“Techniques in Managing Complex Spine Deformities” will be presented by Dr. Steven Mardjetko, MD, FAAP and Dr. Hani Mhaidli, MD, PhD, on Friday, July 18th at 12:15 in Room 5 of the Valencia Conference Centre.
We look forward to seeing you in Spain!
Download as PDF
SpineCraft is a sponsor of the SRS with Diamond Level Support and will be exhibiting at the 2014 IMAST International Meeting.
We invite you to visit the SpineCraft booth at the 2014 IMAST 21st International Meeting on Advanced Spine Techniques, July 16th - 19th in Valencia, Spain, where we will have our latest spine solutions, a Hands-on Workshop, and expert staff on hand to answer your questions.
The Hands-on Workshop, entitled:“Techniques in Managing Complex Spine Deformities” will be presented by Dr. Steven Mardjetko, MD, FAAP and Dr. Hani Mhaidli, MD, PhD, on Friday, July 18th at 12:15 in Room 5 of the Valencia Conference Centre.
We look forward to seeing you in Spain!
Download as PDF
March 20, 2014 | Next Generation of Deformity Correction Technology: The ASTRA Spine System
The ASTRA Spine System is optimized for use with advanced instrumentation developed specifically for complex spine procedures. The ASTRA implants and instruments are simple and precise, providing surgeons with exemplary reliability. The implants are designed with proven technology that allows intraoperative flexibility to change rod diameters and material types while maintaining a low profile and providing exceptional strength.
Download as PDF
The ASTRA Spine System is optimized for use with advanced instrumentation developed specifically for complex spine procedures. The ASTRA implants and instruments are simple and precise, providing surgeons with exemplary reliability. The implants are designed with proven technology that allows intraoperative flexibility to change rod diameters and material types while maintaining a low profile and providing exceptional strength.
Download as PDF
January 28, 2014 | Extended APEX Product Line
SpineCraft has expanded their APEX product line!
The streamlined and versatile line of APEX spinal implants now includes a uniplanar screw option for increased operative adaptability. The APEX Uniplanar Screw combines the strength of a monoaxial screw with the articulation and freedom of a polyaxial screw by restricting movement in the medial/lateral plane while maintaining articulation in the cranial/caudal plane. The APEX Uniplanar Screw’s low profile screw head has features for easy instrument attachment designed to save operating room time.
Download as PDF
SpineCraft has expanded their APEX product line!
The streamlined and versatile line of APEX spinal implants now includes a uniplanar screw option for increased operative adaptability. The APEX Uniplanar Screw combines the strength of a monoaxial screw with the articulation and freedom of a polyaxial screw by restricting movement in the medial/lateral plane while maintaining articulation in the cranial/caudal plane. The APEX Uniplanar Screw’s low profile screw head has features for easy instrument attachment designed to save operating room time.
Download as PDF
December 30, 2013 | SpineCraft Announces Release of VELOX ACP System
The new VELOX Anterior Cervical Plate System coming Q2 of 2014
SpineCraft’s VELOX Anterior Cervical Plate System has been designed to meet wide clinical needs by offering a plate/screw construct with integrated automatic locking mechanism and the choice of fixed or variable angle screws for ease of implantation in different pathologies.
The VELOX Anterior Cervical Plate System will be available for distribution internationally and in the US.
Download as PDF
The new VELOX Anterior Cervical Plate System coming Q2 of 2014
SpineCraft’s VELOX Anterior Cervical Plate System has been designed to meet wide clinical needs by offering a plate/screw construct with integrated automatic locking mechanism and the choice of fixed or variable angle screws for ease of implantation in different pathologies.
The VELOX Anterior Cervical Plate System will be available for distribution internationally and in the US.
Download as PDF
December 18, 2013 | SpineCraft Launches New OsteoPore DBM Products
This month, SpineCraft is launching two new Demineralized Bone Matrix products:
OsteoPore™ Gen-II DBM
OsteoPore™ Allo DBM + Cancellous bone
These products are now available. E-mail or call us for more information or a product brochure.
Download as PDF
This month, SpineCraft is launching two new Demineralized Bone Matrix products:
OsteoPore™ Gen-II DBM
OsteoPore™ Allo DBM + Cancellous bone
These products are now available. E-mail or call us for more information or a product brochure.
Download as PDF
December 11, 2013 | APEX System Clearance
The APEX Spine System recently received regulatory clearance in Australia and in New Zealand. Product launch in these two important markets is planned for early 2014 in collaboration with SpineCraft's distributor, Zimmer Australia and New Zealand.
Download as PDF
The APEX Spine System recently received regulatory clearance in Australia and in New Zealand. Product launch in these two important markets is planned for early 2014 in collaboration with SpineCraft's distributor, Zimmer Australia and New Zealand.
Download as PDF
December 4, 2013 | SpineCraft Has Expanded Again
In early December, SpineCraft opened their new building expansion which has nearly doubled the company facility space. The new added space includes a dedicated workshop & training lab and additional warehouse space.
Download as PDF
In early December, SpineCraft opened their new building expansion which has nearly doubled the company facility space. The new added space includes a dedicated workshop & training lab and additional warehouse space.
Download as PDF
December 4, 2013 | SpineCraft Has Expanded Again
In early December, SpineCraft opened their new building expansion which has nearly doubled the company facility space. The new added space includes a dedicated workshop & training lab and additional warehouse space.
Download as PDF
In early December, SpineCraft opened their new building expansion which has nearly doubled the company facility space. The new added space includes a dedicated workshop & training lab and additional warehouse space.
Download as PDF
October 4, 2013 | SpineCraft Exhibiting at 2013 NASS Annual Meeting
We invite you to visit the SpineCraft booth at the 2013 NASS Annual Meeting, October 9-12 in New Orleans, where we will have our latest spine solutions, in-booth demonstrations and expert staff on hand to answer your questions. Also, call us at 877-731-SPINE (7746) to reserve your exclusive invitation to two hands-on workshops at the New Orleans Downtown Marriott Convention Center:
October 9th, 6-7pm: "The Importance of Implant Choices and Deformity Correction Sequences in Attaining Optimal Radiographic Outcomes in Pediatric and Adult Spinal Deformity"
October 10th, 6-7pm: "Approaches and Techniques for Complex Spinal Deformity Correction in Adolescents and Adults"
The workshops will be presented by Dr. Steven Mardjetko.
We hope to see you there!
Download as PDF
We invite you to visit the SpineCraft booth at the 2013 NASS Annual Meeting, October 9-12 in New Orleans, where we will have our latest spine solutions, in-booth demonstrations and expert staff on hand to answer your questions. Also, call us at 877-731-SPINE (7746) to reserve your exclusive invitation to two hands-on workshops at the New Orleans Downtown Marriott Convention Center:
October 9th, 6-7pm: "The Importance of Implant Choices and Deformity Correction Sequences in Attaining Optimal Radiographic Outcomes in Pediatric and Adult Spinal Deformity"
October 10th, 6-7pm: "Approaches and Techniques for Complex Spinal Deformity Correction in Adolescents and Adults"
The workshops will be presented by Dr. Steven Mardjetko.
We hope to see you there!
Download as PDF
July 10, 2013 | Zimmer Announces Distribution of APEX Spine System
MINNEAPOLIS, MN, July 10, 2013 /PRNewswire/ -- Zimmer Spine, Inc. today announced that the Company has begun distribution of the APEX Spine System, a comprehensive set of implants and instruments designed to safely and effectively correct a broad range of spinal deformities, as well as complex and degenerative spinal diseases. Under the terms of a long-term agreement with SpineCraft (Westmont, IL), Zimmer Spine will exclusively market the APEX Spine System to healthcare organizations across the United State, Canada, Australia and New Zealand.
The APEX Spine System is a pedicle screw fixation system that provides immobilization and stabilization in the thoracic, lumbar and sacral spine regions, in the surgical treatment of acute and chronic spinal instabilities and deformities. Designed for skeletally mature patients as an adjunct to spinal fusion, the APEX Spine System consists of longitudinal rods, pedicle screws, hooks, lateral connectors and cross connectors. With a comprehensive range of component sizes, the APEX Spine System addresses a broad range of spinal pathologies with a low-profile design that allows surgeons to maximize bone graft. The APEX Spine System's custom deformity-correction instruments are designed to shorten surgery time while enabling safer, more controlled correction of spinal deformities.
Download as PDF
MINNEAPOLIS, MN, July 10, 2013 /PRNewswire/ -- Zimmer Spine, Inc. today announced that the Company has begun distribution of the APEX Spine System, a comprehensive set of implants and instruments designed to safely and effectively correct a broad range of spinal deformities, as well as complex and degenerative spinal diseases. Under the terms of a long-term agreement with SpineCraft (Westmont, IL), Zimmer Spine will exclusively market the APEX Spine System to healthcare organizations across the United State, Canada, Australia and New Zealand.
The APEX Spine System is a pedicle screw fixation system that provides immobilization and stabilization in the thoracic, lumbar and sacral spine regions, in the surgical treatment of acute and chronic spinal instabilities and deformities. Designed for skeletally mature patients as an adjunct to spinal fusion, the APEX Spine System consists of longitudinal rods, pedicle screws, hooks, lateral connectors and cross connectors. With a comprehensive range of component sizes, the APEX Spine System addresses a broad range of spinal pathologies with a low-profile design that allows surgeons to maximize bone graft. The APEX Spine System's custom deformity-correction instruments are designed to shorten surgery time while enabling safer, more controlled correction of spinal deformities.
Download as PDF
July 10, 2013 | SpineCraft Exhibit at 20th International Meeting on Advanced Spine Techniques
SpineCraft will be participating this week at the 20th International Meeting on Advanced Spine Techniques at the Vancouver Convention Center in Vancouver, British Columbia. We will be exhibiting at booth #123 from Wednesday, July 10th through Saturday, July 13th. Please stop by our booth to see our expanding Spine Systems line and have us answer any question and share with you the latest information about our systems.
IMAST gathers leading spine surgeons, innovative research, and the most advanced spine technologies in an international forum. For three days, surgeons from around the world will discuss, debate and demonstrate new spine techniques to help each other improve patient care.
Download as PDF
SpineCraft will be participating this week at the 20th International Meeting on Advanced Spine Techniques at the Vancouver Convention Center in Vancouver, British Columbia. We will be exhibiting at booth #123 from Wednesday, July 10th through Saturday, July 13th. Please stop by our booth to see our expanding Spine Systems line and have us answer any question and share with you the latest information about our systems.
IMAST gathers leading spine surgeons, innovative research, and the most advanced spine technologies in an international forum. For three days, surgeons from around the world will discuss, debate and demonstrate new spine techniques to help each other improve patient care.
Download as PDF
April 24, 2013 | SpineCraft is Growing
New Location, New Hires
At the end of 2012, we completed the move to our new office location in Westmont, Illinois and have since been expanding our team.
Zimmer Distribution Agreement for the APEX Spine System
SpineCraft is excited to announce the start of the collaboration with Zimmer Spine in a number of key markets for the distribution of SpineCraft’s Spine System. Zimmer Spine is now the exclusive distributor for the APEX Spine System in the US, Canada, Australia, and New Zealand. This will include the APEX Deformity, Complex Spine, and Degenerative modules.
Download as PDF
New Location, New Hires
At the end of 2012, we completed the move to our new office location in Westmont, Illinois and have since been expanding our team.
Zimmer Distribution Agreement for the APEX Spine System
SpineCraft is excited to announce the start of the collaboration with Zimmer Spine in a number of key markets for the distribution of SpineCraft’s Spine System. Zimmer Spine is now the exclusive distributor for the APEX Spine System in the US, Canada, Australia, and New Zealand. This will include the APEX Deformity, Complex Spine, and Degenerative modules.
Download as PDF
December 24, 2012 | SpineCraft Has Moved
SpineCraft proudly announces our new location as of December 24th, 2012:
777 Oakmont Lane • Suite 200
Westmont, IL 60559
Please note our new numbers:
Phone: 630-920-7300
Fax: 630-920-7310
Toll Free Phone: 877-731-SPINE
Toll Free Fax: 877-741-SPINE
Download as PDF
SpineCraft proudly announces our new location as of December 24th, 2012:
777 Oakmont Lane • Suite 200
Westmont, IL 60559
Please note our new numbers:
Phone: 630-920-7300
Fax: 630-920-7310
Toll Free Phone: 877-731-SPINE
Toll Free Fax: 877-741-SPINE
Download as PDF
April 12, 2012 | SpineCraft launches the AIM MIS Spine System
The first AIM MIS surgeries in the US were a success. Feedback from surgeons has been very positive and they were particularly pleased with instrument performance and the exceedingly small incision required.
The Pistolgrip TLIF Cage Inserter was well-suited to the MIS approach. One of the comments from the first US surgery was "The pedicle screw part of the surgery went quickly and smoothly". Additional one-level and two-levels cases have been scheduled.
Download as PDF
The first AIM MIS surgeries in the US were a success. Feedback from surgeons has been very positive and they were particularly pleased with instrument performance and the exceedingly small incision required.
The Pistolgrip TLIF Cage Inserter was well-suited to the MIS approach. One of the comments from the first US surgery was "The pedicle screw part of the surgery went quickly and smoothly". Additional one-level and two-levels cases have been scheduled.
Download as PDF
October 26, 2011 | SpineCraft Exhibit at the 26th Annual NASS Meeting
Be sure to visit us in booth number 226, where we'll be showcasing our latest offerings.
The NASS Annual Meeting is one of the largest multidisciplinary continuing medical education (CME) meetings for spine specialists in North America.
This multidisciplinary conference is designed for orthopedic surgeons, neurosurgeons, physiatrists, pain specialists, neurologists, radiologists, physical therapists, rheumatologists, anesthesiologists, osteopathic physicians, researchers, nurse practitioners, chiropractors and other physicians and allied health professionals involved in spine care.
Our dedication to providing you with current research and data, promoting discussions of new scientific developments and fostering research and training in the field of spinal disorders makes NASS' Annual Meeting the must attend meeting in spine.
Be sure to visit us in booth number 226, where we'll be showcasing our latest offerings.
The NASS Annual Meeting is one of the largest multidisciplinary continuing medical education (CME) meetings for spine specialists in North America.
This multidisciplinary conference is designed for orthopedic surgeons, neurosurgeons, physiatrists, pain specialists, neurologists, radiologists, physical therapists, rheumatologists, anesthesiologists, osteopathic physicians, researchers, nurse practitioners, chiropractors and other physicians and allied health professionals involved in spine care.
Our dedication to providing you with current research and data, promoting discussions of new scientific developments and fostering research and training in the field of spinal disorders makes NASS' Annual Meeting the must attend meeting in spine.
July 29, 2011 | APEX Spine System Cannulated Screws FDA Clearance
SpineCraft is pleased to announce the FDA clearance of the APEX Spine System Cannulated Screws for use with the AIM Minimally Invasive Surgery Instrumentation. SpineCraft is planning the release of the MIS system in the US and Europe later this quarter for degenerative lumbar applications and in early Q1-2012 for deformity thoracolumbar applications. The AIM MIS System is one of the first minimally invasive systems in the market available with 4.75mm screws.
Download as PDF
SpineCraft is pleased to announce the FDA clearance of the APEX Spine System Cannulated Screws for use with the AIM Minimally Invasive Surgery Instrumentation. SpineCraft is planning the release of the MIS system in the US and Europe later this quarter for degenerative lumbar applications and in early Q1-2012 for deformity thoracolumbar applications. The AIM MIS System is one of the first minimally invasive systems in the market available with 4.75mm screws.
Download as PDF
March 18, 2011 | ALTUM Anterior Cervical Plate System FDA Clearance
SpineCraft is pleased to announce the FDA clearance of the ALTUM Anterior Cervical Plate System.
Download as PDF
SpineCraft is pleased to announce the FDA clearance of the ALTUM Anterior Cervical Plate System.
Download as PDF



